.: Crystal Forms
Minerals At Their Best
Crystals are minerals that have had the chance to grow in the shape that they were meant to be. Just like your DNA determines the color of your eyes, how tall you will get to be and the shape of your bones, the chemicals that a mineral is made of determines what shape it gets to be. We can tell different minerals apart by what crystal shape they are.
A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Because there are repeated units, crystals have recognizable structures. There are seven systems of crystal structures, which are also called lattices or space lattices.
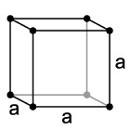
| 1. Cubic or Isometric - not always cube shaped! You'll also find octahedrons (eight faces) and dodecahedrons (10 faces). |

Halite as rock salt has a cubic structure. |
|
The simple cubic system has one lattice point on each corner of the cube with each lattice point shared equally between eight adjacent cubes. |
| 2. Tetragonal - similar to cubic crystals, but longer along one axis than the other, forming double pyramids and prisms. |

Wulfenite has a tetragonal structure. |
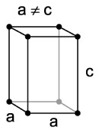 |
Tetragonal crystal lattices result from stretching a cubic lattice along one lattice vectors, making the cube a rectangular prism with a square base. |
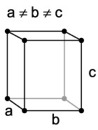
| 3. Orthorhombic - like tetragonal crystals except not square in cross section (when viewing the crystal on end), forming rhombic prisms or dipyramids (two pyramids stuck together). |

Aragonite has a orthorhombic structure. |
|
Orthorhombic lattices are made by stretching a cubic lattice along two lattice vectors by two factors, forming a rectangular prism with a rectangular base. All three bases intersect at 90 degree angles and the three lattice vectors are mutually orthogonal. |
| 4. Hexagonal - six-sided prisms. When you look at the crystal on-end, the cross section is a hexagon. |

Beryl has a hexagonal crystal structure. |
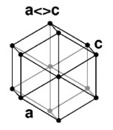 |
A hexagonal lattice has the same symmetry as a right prism with a hexagonal base. Graphite is an example of a hexagonal crystal. |
| 5. Trigonal - possess a single 3-fold axis of rotation instead of the 6-fold axis of the hexagonal division. |

Quartz has a trigonal crystal structure. |
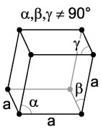 |
The rhombohedral (or trigonal) crystal is described by vectors of equal length, all three of which are not mutually orthogonal. You can think of the rhombohedral system as the cubic system stretched along a body diagonal. |
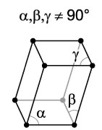
| 6. Triclinic - usually not symmetrical from one side to the other, which can lead to some fairly strange shapes. |

Copper sulfate has a triclinic crystal structure. |
|
A triclinic crystal is described by vectors of unequal length. All three vectors are not mutually orthogonal. |
| 7. Monoclinic - like skewed tetragonal crystals, often forming prisms and double pyramids. |

Orthoclase has a monoclinic crystal structure. |
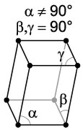 |
A monoclinic lattice is described by vectors of unequal length that form a rectangular prism with a parallelogram as base. Two pairs of vectors are perpendicular, while the third pair makes an angle other than 90 degrees. |
This is a very simplified view of crystal structures. In addition, the lattices can be primitive (only one lattice point per unit cell) or non-primitive (more than one lattice point per unit cell). Combining the 7 crystal systems with the 2 lattice types yields the 14 Bravais Lattices (named after Auguste Bravais, who worked out lattice structures in 1850). The structure of real crystals is pretty complicated!
Sometimes minerals form in spaces where there is not a lot of room, so they don't have a crystal shape. When there is just a big hunk of a mineral, it is called a massive mineral. If there is a definite shape with easy to see flat sides, it is called a mineral crystal.
Most of the earth's crystals were formed millions of years ago. Crystals form when the liquid rock from inside the earth cool and harden. Sometimes crystals form when liquids underground find their way into cracks and slowly deposit minerals. Most mineral crystals take thousands of years to "grow" but some like salt (halite) can form so quickly that you can watch them grow at home!
There are four main categories of crystals, as grouped by their chemical and physical properties:
- Covalent Crystals - A covalent crystals has true covalent bonds between all of the atoms in the crystal. You can think of a covalent crystal as one big molecule. Many covalent crystals have extremely high melting points. Examples of covalent crystals include diamond and zinc sulfide crystals.
- Metallic Crystals - Individual metal atoms of metallic crystals sit on lattice sites. This leaves the outer electrons of these atoms free to float around the lattice. Metallic crystals tend to be very dense and have high melting points.
- Ionic Crystals - The atoms of ionic crystals are held together by electrostatic forces (ionic bonds). Ionic crystals are hard and have relatively high melting points. Table salt (NaCl) is an example of this type of crystal.
- Molecular Crystals - These crystals contain recognizable molecules within their structures. A molecular crystal is held together by non-covalent interactions, like van der Waals forces or hydrogen bonding. Molecular crystals tend to be soft with relatively low melting points. Rock candy, the crystalline form of table sugar or sucrose, is an example of a molecular crystal.
As with the lattice classification system, this system isn't completely cut-and-dried. Sometimes it's hard to categorize crystals as belonging to one class as opposed to another. However, these broad groupings will provide you with some understanding of structures. I'll test your knowledge by referring to these crystal shapes in crystal-growing tutorials!
Some people think of crystals as clear pretty rocks that are used for jewelry. Amethyst is a very common quartz crystal. Crystals do not have to be clear, but those are the kinds you will usually see in the stores.
"Introduction to Crystallography and Mineral Crystal Systems", written by Mike and Darcy Howard, provides information about crystal structures, formations, and classification great detail.
Come join us on our Facebook Page
|




